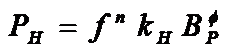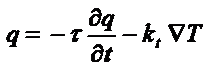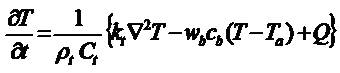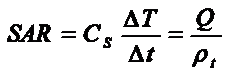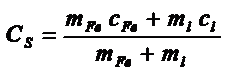
|
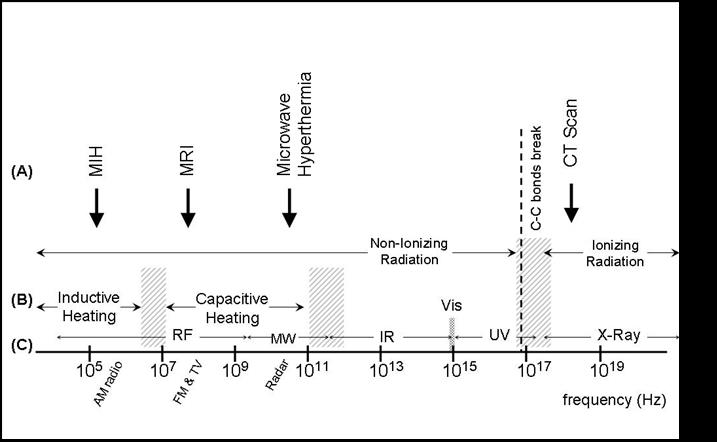
The process involved in the Magnetic Hyperthermia, which is based on the known hyper sensibility of tumor cells to heating (hyperthermia), is related to energy dissipation when a ferromagnetic material is placed on an external alternating magnetic field. The MFH is the idea of attaining cytolysis of specific tumor tissues by hyperthermia, through the magnetic losses of sub-domain magnetic particles when an alternating ( ac) magnetic field is applied. The figure below shows the ranges of frequencies involved in different medical therapies using electromagnetic radiation, as well as the physical processes (bottom) occurying for each frequency range.
|
|
To identify the mechanisms of magnetic losses in nanostructured materials in physiological conditions should be the first step towards the design of therapeutic materials to be used in biological areas. |
|
Frequency ranges for (A) some of the most used diagnostic/therapy equipments for clinical protocols, and (B) the main physical mechanisms at each frequency range. Also shown in (C) is the common nomenclature for the electromagnetic waves at each region: RF = radiofrequency; MW = microwaves; IR = infrared; Vis = visible; UV = ultraviolet and X-Ray. |
|
Clearly, the success of such approach depends critically of the ability to specifically attach a given particle on a certain type of cells, those who are to be killed. This is a quite complex biochemical, biological and medical issue, though many groups are working on it. Other issues to be solved (depending on the kind of organs to be treated) are: to transport to the target, to cheat the body immune system, to minimize the mass of magnetic material, to detect possible accumulation of magnetic material in other organs. Regarding the energy loss at the MNPs, there are two different effects to be considered: a) magnetic losses through spin-flipping of the single-domain magnetic moment (called Néel losses); and b) energy loss from mechanical rotation of the particles, acting against viscous forces of the liquid medium (Brown losses). For details of these two mechanisms, go HERE For clinical applications, the granular materials should present low levels of toxicity, as well as a high saturation magnetic moment in order to minimize the doses required for temperature increase. In this context, magnetite (Fe3O4) is a promissing candidate because presents a high Curie temperature (TC), high saturation magnetic moment (90-98 emu/g, or ~450-500 emu/cm3), and has shown the lowest toxicity index in pre-clinic tests. Although from the point of view of synthesis the material is cheap and can be obtained with high purity from relatively easy routes, the fabrication of MNPs with high structural order, and having few nanometer diameters is intrinsically complicated since the high surface/volume ratio makes the surface disorder effects to be important. |
|
Hyperthermia |
|
Hyperthermia in detail |
|
The study of heat generation under ac fields has been historically related to electrical machines, seeking to minimize power losses to improve the performance of electro-mechanic conversion devices (motors, transformers, etc…). The simulation and measurement of magnetic losses in magnetic materials (mostly iron and its alloys NiFe and SiFe) have been developed along the last years for high-performance electromechanical devices subjected to adverse working conditions of high frequencies and harmonics contents. Power losses in these materials are considered as the sum of two components, PT = PF + PH, where PT are the losses due to eddy (Foucault) currents and PH are the hysteretic losses, related to domain creation, propagation and extinction inside the material. Equations for eddy currents are solved by conventional electromagnetic theory. However, hysteresis loss has a nonlinear behavior:
where f is the frequency in Hz, BP is the peak induction and kH, n and f are adjustable parameters depending of each type of material. This empirical expression proposed by Steinmetz more than 100 years ago is sill used for manufacturers to estimate the hysteretic losses at the induction values of the machine’s operating regime. The search for new materials with better performance yielded the incorporation of nanostructured composites into the magnetic core of AC motors and transformers, resulting in a better understanding of the microscopic mechanisms involved in magnetic losses of nanostructured phases. Unfortunately, the situation regarding power losses in MNPs for biological applications is quite different from the described above: systematic studies on the mechanisms of power losses in magnetic colloids are rather scarce, and a systematic body of measurements in MNPs under physiological conditions is still lacking.
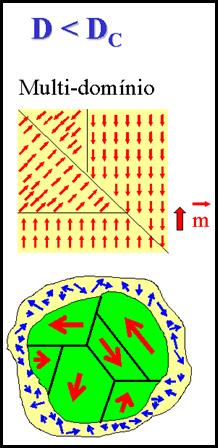
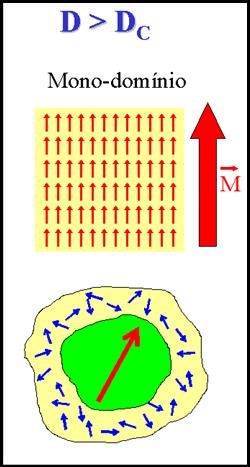
For a piece of metal subjected to low- and medium-frequency alternating fields (> 102 – 103 Hz, for example the case of nuclei of electrical motors) the main mechanism of power losses is parasitic currents (eddy currents). On the other hand, in ceramic materials the dissipation of power is mainly originated in processes of nucleation, growth and extinction of magnetic domains. For single-domain particles in physiological conditions the situation differs radically, because a) the magnetic saturation is reached by coherent rotation of the total magnetic moment of each particle; and b) the hysteresis cycles are theoretically reversible and thus they do not entail magnetic losses. In addition to coherent rotation to be considered for single-domain particles, physiological conditions allow mechanical rotation of the particles as a response to the external magnetic field, at least for low frequencies . It follows that for colloidal dispersions the analysis of the heat transference processes must include the effects of both the Brownian motion and fluid. It is clear that the mechanisms of power losses in colloids must be identified before new, more efficient therapeutic materials can be designed to maximize the generate heat for MFH applications.
Once the heat is generated at the magnetic particles, the problem of heat propagation in living matter becomes an important one. Originally, the heat flux propagated in non-homogeneous materials such as biological tissues have been studied in meat-containing food products. In these models the thermal propagation wave is described using an hyperbolic heat transfer model [36], characterized by a thermal relaxation time, of the order of 20–30s, and a differential equation governing the propagation speed of the heat flux, q, given by
where t and kt are the thermal relaxation time and thermal conductivity, respectively. Applying the above model to real situations of living organisms requires the inclusion of the system’s response to local heating, mainly blood perfusion that acts as a efficient refrigerating system in highly vascularised tumors. This is done by using Pennes’ equation to estimate the temperature field T(x,y,z,t) at nearby tissues
where rt and Ct are the density and the specific heat capacity of the tissue, respectively, Q is the density of heat production rate, Ta is the temperature at infinite distances, and wb, cb are the perfusion rate and specific heat capacity of blood, respectively. Given all the relevant physical parameters and eq. III, the heat distribution in any internal element of the body could be calculated by solving the inverse heat transfer problem. However, the complex boundary conditions associated to internal organs is the major obstacle for the use of this model. Nevertheless, heat generating diagnostic tools such as high intensity focused ultrasound (HIFU) and MRI have used this approach with some success to evaluate potential hazards and to establish tolerance limits for clinical protocols. A complementary approach has been reported by Andrä et al., who made numerical simulations of the spatial temperature distribution during exposure to ac magnetic fields with the physical parameters close to the experimental clinical situation for breast carcinoma, and compared the results with in vitro experiments .
The heating capacity of a magnetic material or electromagnetic device is quantified through the specific absorption power rate (SAR), defined as the amount of energy converted into heat per time and mass . In terms of the usual experiments and parameters for magnetic colloids, the loss power per gram of Fe3O4 is obtained from the heating curves within the initial DT temperature rising interval through the definition
where CS is the sample heat capacity, defined as a mass-weighted mean value for a given concentration of magnetic material, calculated as
with cFe, mFe and cl, ml being the specific heat capacities and masses of magnetic material and liquid carrier, respectively. The last member shows the relationship between the functional definition of SAR and eq. III. From the instrumental point of view, there is a need of innovative equipments for measuring the many phenomena involved in power losses in fluidic samples . For example, measuring the SAR of a biocompatible colloid under physiological conditions requires generating RF waves with variable frequencies and applied fields, mounted around an appropriate Dewar to keep the experiment adiabatic, with the corresponding calorimetric measuring system. |
|
Heat generation |




|
|
As mentioned above, we are usually dealing with two additive mechanisms for energy loss in a nanoparticle suspension: the Néel losses through domain wall displacements and Brown losses from mechanical rotation of the particles in the liquid medium.. For details of these two mechanisms, go here. Te exact nature of the microscopic mechanisms that produce the intracellular hyperthermia are not yet free of discussion. Some doubts have been risen about even the existence of intracellular hyperthermia effects at all. It is well known that the mechanism of magnetic losses in metals (for example, at the metallic nucleus of any electrical motor) subjected to ac currents of high frequencies (>103 - 105 Hz), is mainly due to parasitic currents (Foucault's currents). On the other hand, power dissipation in ferromagnetic materials (which are usually insulating) is due to nucleation, growth and extinction of magnetic domains. The situation in MNPs of biological applications is quite different from the described above: For particles of less than few nanometers of diameter, the existence of domains is no longer energetically favorable, thus a single-domain is formed inside the particle. Additionally, in physiological conditions the medium is usually liquid, so the existence of mechanical rotation of the particles as a response to the external magnetic field has to be considered, at least for low frequencies. Therefore, in the case of ferrofluids or any colloidal dispersion of MNPs, the analysis of heat transfer processes must include the effects related to Brownian movement and fluid torques.
|
|